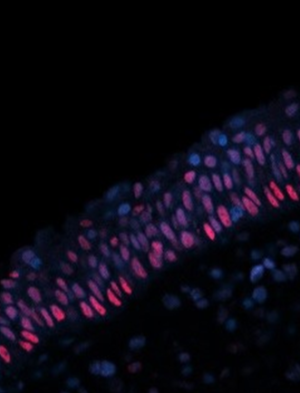
The airway epithelium plays a pathobiologic role in orchestrating inflammation and undergoes extensive remodeling in airway disease. Through airway epithelial cell cultures, single cell RNA-Seq from the human sino-nasal mucosa, and murine models of type 2 lung inflammation we have found once aberrant epithelial cell functions are established, they persist in part due to epigenetically altered epithelial progenitors (1). Additionally, we have identified a novel feature of airway remodeling, the expansion of brush cells. This unique chemosensory epithelial cell population generates IL-25 (2) and cysteinyl leukotrienes (3) to promote type 2 immunity. Our ongoing interest is to understand the pathways driving airway epithelial cell development and function in health and disease.
- Allergic Inflammatory Memory in Human Respiratory Epithelial Progenitor Cells. Ordovas-Montanes et al. Nature. 2018
- The Cysteinyl Leukotriene 3 Receptor Regulates Expansion of IL-25-Producing Airway Brush Cells Leading to Type 2 Inflammation. Bankova et al. Sci Immunol. 2018
- Airway Brush Cells Generate Cysteinyl Leukotrienes Through the ATP Sensor P2Y2. Ualiyeva et al. Sci Immunol. 2020
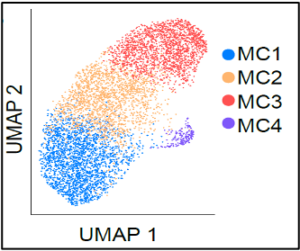
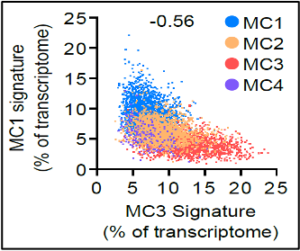
Mast cells are unique tissue-resident immunocytes that generate a wide repertoire of active mediators including cysLTs, amines, proteases, and cytokines. The immune functions of these ancient cells, that preceded adaptive immunity, are poorly understood and the pathways for their tissue expansion and activation in disease remain unclear. We have used transcriptional methods (1-3) and mouse models of allergic disease (4-6) to understand their contribution to tissue homeostasis, host defense, and inflammation.
- Expression Profiling of Constitutive Mast Cells Reveals a Unique Identity within the Immune System. Dwyer et al. Nat Immunol. 2016
- Allergic Inflammatory Memory in Human Respiratory Epithelial Progenitor Cells. Ordovas-Montanes et al. Nature. 2018
- Human Airway Mast Cells Proliferate and Acquire Distinct Inflammation-Driven Phenotypes During Type 2 Inflammation. Dwyer DF et al. Sci Immunol. 2021
- Leukotriene E4 Elicits Respiratory Epithelial Cell Mucin Release Through the G-Protein-Coupled Receptor, GPR99. Bankova et al. Proc Natl Acad Sci. 2016
- Type 2 Cysteinyl Leukotriene Receptors Drive Aspirin Sensitivity and Eosinophilic Immunopathology. Liu et al. J Immunol. 2018
- Lineage-Specific Regulation of Inducible and Constitutive Mast Cells in Allergic Airway Inflammation. Derakhshan et al. J Exp Med, 2021
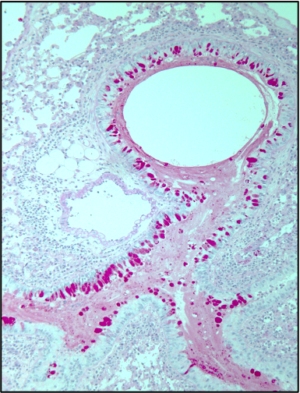
The laboratory has a long-standing interest in defining the immune pathways activated by common environmental insults such as dust mite and mold aeroallergens. Through fractionation of crude allergen extracts, signaling inhibition, and molecular approaches, we have defined key signaling pathways through which tissue-resident innate immune cells sense the external environment to pattern adaptive immunity. This includes a conserved role for the enzyme leukotriene C4 synthase (LTC4S) which is activated in dendritic cells (1) and mast cells (2), among other cell types, and generates pro-inflammatory lipid mediators, cysteinyl leukotrienes (cysLTs). We have discovered that cysLT generation can be elicited by allergen-associated glycan recognition by the C-type lectin receptor, Dectin-2 (1, 3), and that this recognition plays a key role in both priming for Th2/Th17 immunity (4). Our ongoing work includes defining additional innate immune pathways in the response to aeroallergens.
- Dectin-2 Recognition of House Dust Mite Triggers Cysteinyl Leukotriene Generation by Dendritic Cells. Barrett et al. J Immunol. 2009
- Leukotriene E4 Elicits Respiratory Epithelial Cell Mucin Release Through the G-Protein-Coupled Receptor, GPR99. Bankova et al. Proc Natl Acad Sci USA. 2016
- Dectin-2 Mediates Th2 Immunity Through the Generation of Cysteinyl Leukotrienes. Barrett et al. J Exp Med. 2011
- Phosphoinositide 3-Kinase δ Regulates Dectin-2 Signaling and the Generation of Th2 and Th17 Immunity. Lee et al. J Immunol. 2016